How to Find Ratio of Protonated to Deprotonated
ColorbluepK_a - logK_a where K_a - the acid dissociation constant of the weak acid. In this case log A - HA 0 and A - HA 1.
Pka of each is given as.

. A 0120 M CHCH OHCOOH aq lactic acid b 14 10M CHCH OH COOH aq c 015 M NHCI aq d 015 m NaCHCO aq and e 0112 m CHN aq trimethylamine. The deprotonated form of the R group of cysteine. A list of the biology- medicine- and MIT research-related examples used in 5111 is provided below.
100 106 192 SolutionInn. At a pH of 700 the ratio of protonated His molecules to deprotonated His molecules is 101. For example if the pKa of the acid is 475 at a pH of 475 that acid will exist as 50 protonated and 50 deprotonated.
The ratio of the protonated to the deprotonated form of the acid is 100. 10 of 23 Now use the Henderson-Hasselbalch equation to estimate the ratio of protonated versus deprotonated histidine His molecules at a pH of 700. -The solution pH 5 is greater than pKa1 which means the first acidic proton has been fully deprotonated.
ColorbluepH pK_a log conjugate baseweak acid Here pK_a is equal to. A- Hence the correct answer is 968. Given pH 60.
This equation is very useful for calculating the ratio of the unprotonated to the protonated form of the acid. Determine acidbase ratio of a buffer. To calculate the ratio of the protonated to the deprotonated form of the acid we are using Henderson Hesselbach equation.
Thus we can see that in a situation in which we have equal amounts of the protonated and deprotonated forms of a conjugate pair the pH pKa. Science Chemistry QA Library Calculate the pH pOH and fraction of solute protonated or deprotonated in the following aqueous solutions. A- pH pKa log HA 11.
Deprotonated A - species for the following compounds at pH 2 5 9 and 14. The pH of stomach 20 Based on the Henderson-Hasselbalch equation. Calculate the ratio of proton acceptor to proton donor at pH 70.
Find the ratio of formate to formic acid when the pK 37 at a pH of 36. Rm pH pK_a logleft A- over HAright where the pKa -log K a. F HA HAA T and the fraction that is deprotonated.
We call it a base because if the given compound is deprotonated then it is a proton donor and by BrønstedLowry definition the proton donor is the acid in an acid-base reaction. Given a pH and pK a of an acid calculate the fraction of the acid that is protonated. One particular case is routinely used in biochemistry.
10 In-HIn A λ2 A λ1acidic A λ1 A λ2basic Bromthymol blue acts as a weak acid in solution. It can thus be in protonated or deprotonated form appearing yellow or blue respectively. Find the pH of a formic acid solution which.
Click on the associated PDF for more information on each example. ZA pH pka log HA Recall that the pKa of the His side chain is 600. Defining R A-HA pH pK a logA-HA.
A T HA A-. By taking the log of the equilibrium expression we find. Select all the pH values at which the protonated form of the R group would predominate.
For more information about ph refer to the link-. PH pKa log A- HA where A - represents the deprotonated form and HA represents the protonated form. As the mathrmpH and mathrmpK_mathrma get further apart then we continue to change this ratio by orders.
For the transfer of protons within the catalytic site of enzymes due to the presence of significant amounts of both the protonated and deprotonated forms of its side chain at biological pH. A- 01072 HA In order to find the percentages let us assume that A- HA 100. Recommended textbook explanations.
Clearly if the mathrmpH differs from the mathrmpK_mathrma by only 1 mathrmpH unit we dont really have a completely protonated or deprotonated molecules but its certainly mostly protonated or deprotonated. Ratio of protonated form deprotonated form Aspirin is a weak acid it contains carboxylic acid group -COOH so when protonated the H is still attached to the acid while deprotonated means H. Since the product of the H 3 O and the ratio NH 3 NH 4 is a constant the larger the H 3 O the smaller the ratio.
PKa1 -log Ka1 215 and pKa2 -log Ka2 720 and pKa3 -log Ka3 1235. 100 1 rating Answer 12 Given. PH 775 pKa 35 To find.
One oft-cited solution to this equation is obtained by arbitrarily setting pH pKa. By recogniz- ing that the sum of these two forms A- HA must equal the total A one can solve simultaneous equations and calculate the. The most common form of the Hendeson - Hasselbalch equation allows you to calculate the pH of a buffer solution that contains a weak acid and its conjugate base.
We are considering a solution with a given pH which means that the H 3 O is known. - The pH is not greater than pKa2 or pKa3 which means the. The ratio of CB WA 1 and according to the HH equation pH pKa log1 or pH pKa.
The ratio In-HIn at any pH can be obtained by dividing equation 8 by equation 9 to give. A- deprotonated form. First of all deprotonation means removing the most acidic proton of the compound by a base that you need to choose.
Answer to What is the ratio of protonated HA vs. We are interested in the ratio of the deprotonated form to the protonated form NH 3 NH 4. Now put all the given values in this expression we get.
So we can visualize the task as such we need something a base to react with the. 37 rows These in-class examples range from two to ten minutes designed to succinctly introduce biological connections without sacrificing any chemistry content in the curriculum. Electric Power Find the current in a 24 W.
F A- A-A T where A T is the total concentration of acid. HA protonated form. This means that when the pH is equal to the pKa there are equal amounts of protonated and deprotonated forms of the acid.
01072HA HA 100. Science Chemistry QA Library In other words we use the Henderson-Hasselbalch equation below to determine the ratio of protonated vs deprotonated molecules in the solution and therefore the molecules overall charge. The ratio of protonated to deprotonated form depends on the pKa of the R group and the pH of the solution.

Henderson Hasselbalch Equation
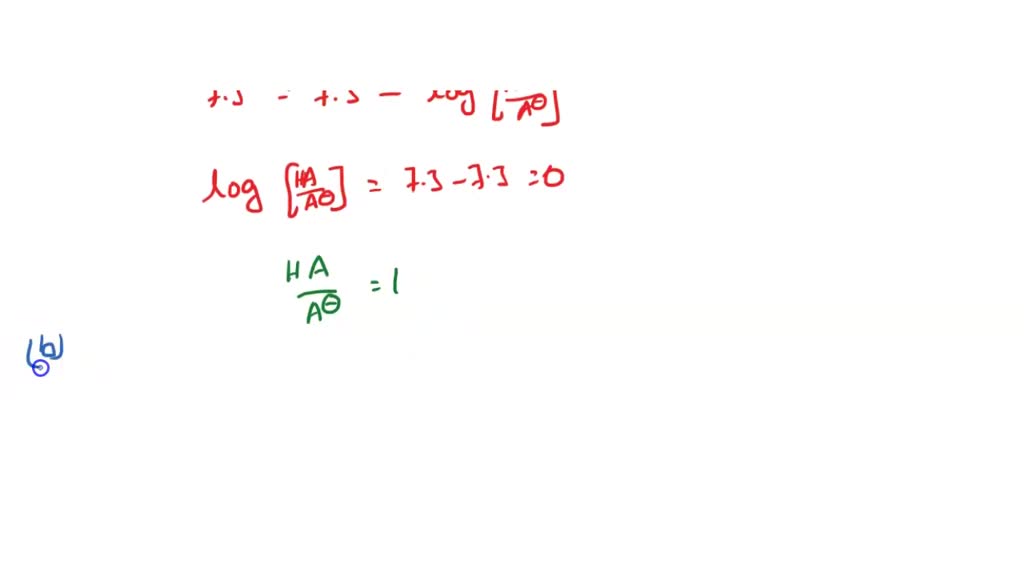
Solved 8 You Are Investigating Molecule That Has The Potential To Be Cancer Drug The Pka Of This Compound Is 7 3 A Calculate The Ratio Of Protonated To Deprotonated Compound At Ph 7 3

No comments for "How to Find Ratio of Protonated to Deprotonated"
Post a Comment